Vero Cell Vaccine Covid-19 Effectiveness | Sinopharm S Covid 19 Vaccine Shows 86 Efficacy Uae Health Agency Says Biospace
The World Health Organization on Tuesday approved the Sinovac Covid-19 vaccine for emergency use -- the second Chinese jab to receive the WHOs green light. All confirmed cases are all confirmed by DSMB blind examination.
Coronavirus Covaxin Efficacy Is 81 Works Against Variants The Hindu
One of the earliest papers to identify the pathogen behind the 2003 SARS outbreak for instance used this kind of cell.

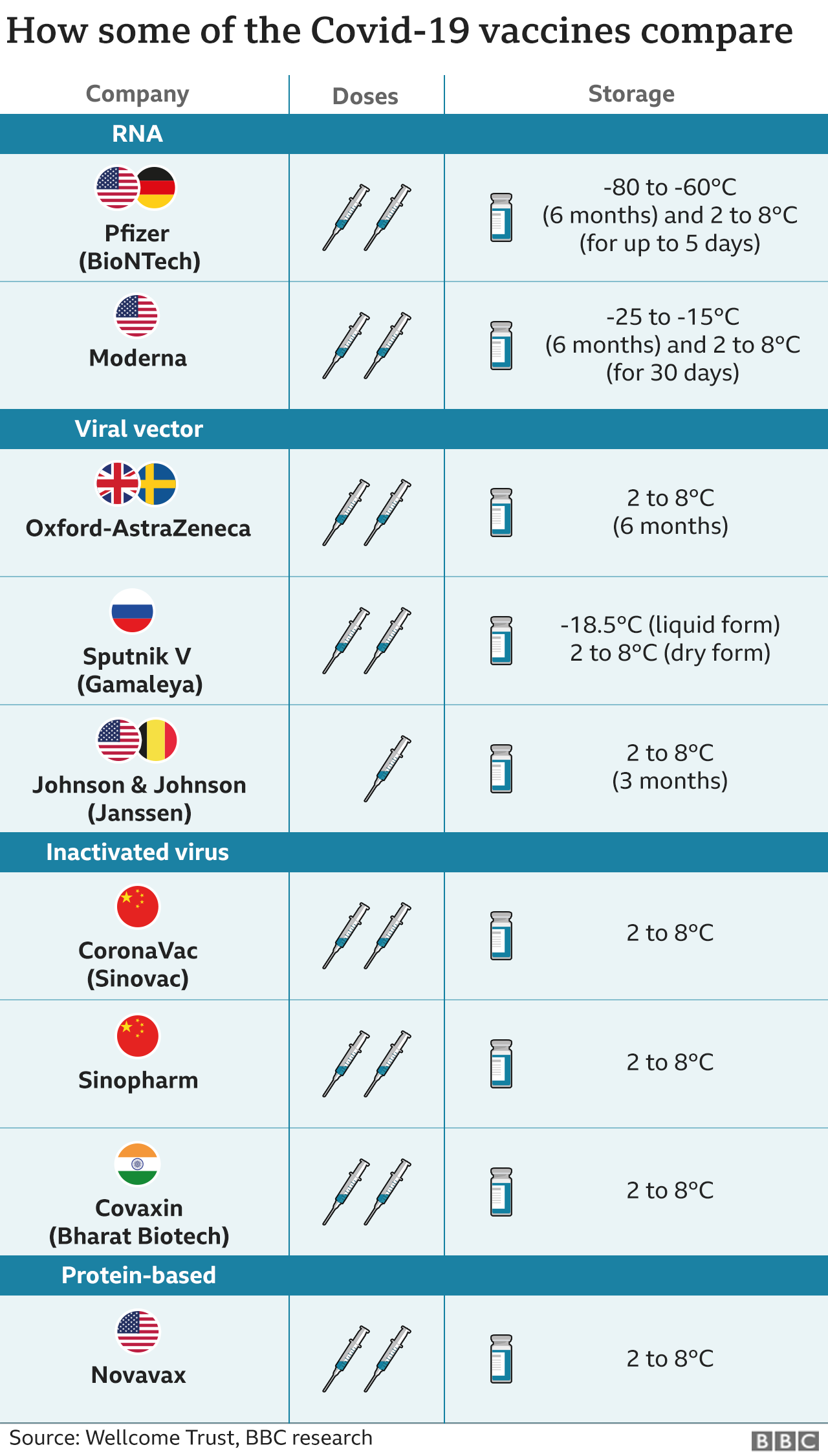
Vero cell vaccine covid-19 effectiveness. Protective effect against COVID 19 after 14 days following the full course of vaccination among healthy population aged 18 years old and above. Its easy storage requirements make it highly suitable for low-resource settings. Table 1 shows the overall vaccine efficacy of the licensed vaccines after completed vaccination.
The SARS-CoV-2 Vaccine VeroCell is an inactivated vaccine against coronavirus disease 2019 COVID-19 which stimulates the bodys immune system without risk of causing disease. COVID-19 Vaccine Vero Cell Inactivated detailed edition Created Date. Vaccine Efficacy 8350 CI 95 6542-9212 Based on symptomatic and RT-PCR positive COVID-19 cases after 14 days and more after the 2nd dose Number of subjects after 14 days and more after the 2nd dose Based on calculation person x year in the follow up period Treatment Arm Number of hospitalized COVID-19 Cases Total Subject Number Person year.
Once inactivated viruses get presented to the bodys immune system they stimulate the production of antibodies and make the body ready. A study by Public Health England PHE found in May the Pfizer PFEN -BioNTech 22UAyDE vaccine was 88 effective against symptomatic disease. It is used for preventing COVID-19 caused by SARS-CoV-2 infection and suitable for people aged 18 years and over for immunization.
On December 29 2020 Sinopharm reported 79 efficacy in an interim evaluation. As 1 July 2021 six of the 71 COVID-19 deaths in Seychelles were among the fully vaccinated people. A Study to Evaluate The Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell in Healthy Population Aged 18 Years Old and Above COVID-19 The safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
While AstraZeneca claimed that the vaccine was 70-percent effective it was later disclosed that the effectiveness was 62 percent in people. Comparison of the proportions of confirmed Covid-19 cases in the two vaccine groups and the placebo group. COVID-19 vaccines licensed for use in the EUEEA have been shown during clinical trials to be highly effective in providing protection against symptomatic COVID-19 and severe disease.
Ema - ema will assess the compliance of covid-19 vaccine vero cell inactivated with the usual eu standards for effectiveness safety and quality. China joined this club of elites of science following the Chinese FDA approval of Sinopharm the subsidiary of state-owned China National Pharmaceutical Group- CNPG first COVID-19 vaccine inactivated Sars-Cov-2 based on the results of the phase-3 clinical trial in UAE and Bahrain showing up to 86 efficacy of the vaccine in preventing COVID-19. The use of cell-culture technologies for the manufacture of influenza vaccines might contribute to improved strain selection and robust vaccine supplies.
When a person is given the vaccine their immune system identifies the inactivated virus as foreign and makes antibodies against it. COVID-19 Vaccine Vero Cell Inactivated also contains an adjuvant a substance that helps strengthen the immune response to the vaccine. The efficacy rating followed an interim report of ongoing human trials conducted in that country CNBC reported.
Vero cells have at times been indispensable to the study of coronaviruses. CoronaVac is an inactivated vaccine to be administered intramuscularly as a course of 2 doses each dose of 05ml contains 600SU inactivated SARS-CoV-2 virus as antigen at an interval of 28 days for routine immunization. We investigated the safety immunogenicity and protective efficacy of a Vero-cell-culture-derived influenza vaccine and assessed the correlation between vaccine efficacy and haemagglutination inhibition antibody titre.
The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell. So far Vero is the only Chinese vaccine for which the manufacturer has published official data. A large phase 3 trial in Brazil showed that two doses administered at an interval of 14 days had an efficacy of 51 against symptomatic SARS-CoV-2 infection 100 against severe COVID-19 and 100 against hospitalization starting 14 days after receiving the second dose.
Secondary Outcome Measures. The COVID-19 vaccine under development by Chinas Sinopharm is showing efficacy of 86 health authorities from the United Arab Emirates reported this morning. In December 2020 UAEs Ministry of Health and Prevention previously announced interim analysis showing BBIBP-CorV to have a 86 efficacy against COVID-19 infection and nearly 100 efficacy in preventing moderate and severe cases.
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5
Seychelles Brings Back Curbs Despite Vaccination Success Bbc News
Sinopharm S Covid 19 Vaccine Shows 86 Efficacy Uae Health Agency Says Biospace
Doubts Over China Vaccines Effectiveness Mar Production Push Nikkei Asia
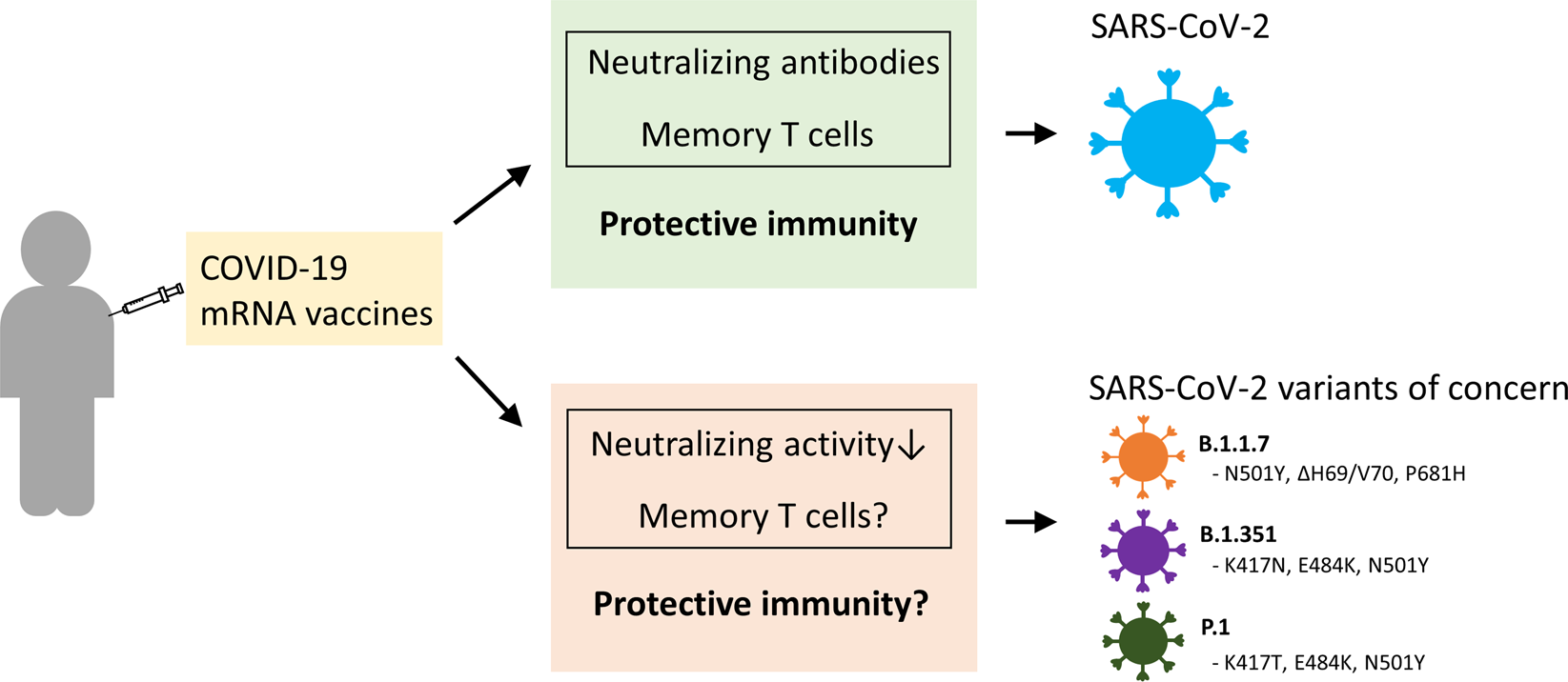
Sars Cov 2 Mutations Vaccines And Immunity Implication Of Variants Of Concern Signal Transduction And Targeted Therapy
Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News
Expert Chinese Vaccines Effective Against Covid 19 Delta Variant Cgtn

Covid 19 Chinese Official Says Homegrown Vaccines Not Very Powerful Euronews
Efficacy Of Sinopharm S Covid 19 Vaccines Proved Again In New Trials Cgtn
Who Lists Sinopharm S Covid 19 Vaccine For Emergency Use
Coronavirus Who Approves Sinovac Covid Vaccine For Emergency Use News Dw 01 06 2021

Sinopharm Vero Cell Inactivated Covid 19 Vaccine
Singapore Excludes Sinovac Shots From Covid 19 Vaccination Tally Nikkei Asia
China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says
World Health Organization Who What S The Difference Between Covid 19 Vaccine Efficacy And Effectiveness Vaccine Efficacy Refers To How The Vaccine Performs In Ideal Conditions Controlled Clinical Trials Vaccine
China S Vaccine Diplomacy Assumes Geopolitical Importance Merics
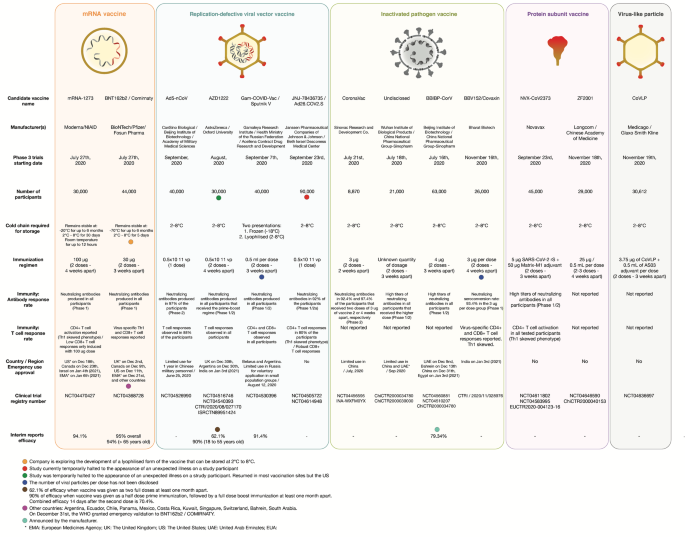
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines

Two Covid 19 Vaccines Approved In China In Less Than 24 Hours 2021 03 02 Bioworld
.JPG)
